Product details
BD® Blunt Plastic Cannula are specifically designed for use with preslit injection sites and vials designed for needleless access. They are not compatible with conventional injection sites that are not preslit. The BD® Blunt Plastic Cannula is intended for use with Abbott LifeShield®, McGaw SafeLine™ and Baxter InterLink® Systems.
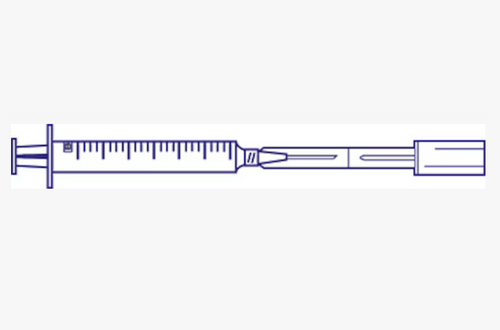
The BD Twinpak™ Dual Cannula Device (Ref. 303390) is designed to support safe, efficient medication preparation and transfer using a closed system. Featuring a dual cannula design, Twinpak™ allows fluid to be transferred between containers without exposing medication to the environment, helping reduce the risk of contamination.
This device is commonly used when reconstituting medications, transferring diluents, or mixing solutions between vials and containers. The closed-system design helps protect both healthcare workers and medications while improving workflow efficiency and consistency.
Ideal for use in hospitals, pharmacies, compounding areas, clinics, and laboratory settings, the BD Twinpak™ dual cannula device supports best practices for medication safety and infection control.
Key Features:
-
Dual cannula device for closed-system fluid transfer
-
Helps reduce contamination risk during medication preparation
-
Supports safe reconstitution and solution transfer
-
Improves workflow efficiency and consistency
-
Designed for professional healthcare use
-
Suitable for hospitals, pharmacies, clinics, and labs
-
Trusted BD Twinpak™ quality
-
Reference Number: 303390