Product details
This simulated medication is used to teach students the proper treatment of asthmatic patients.
- Therapeutic class: Bronchodilator
- Strength: Fluticason Propionat 250 mcg/ Salmeterl 50 mcg
- Unit can be reset back to zero
- Use in scenarios where a powder inhaler is indicated
- The Demo Dose® diskus does not contain powder or APIs
- Packaged in zipper-top bag
- Can be reused
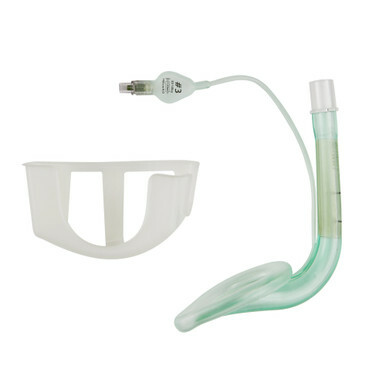
The Demo Dose® Fluticasone/Salmeterol Disk Inhaler 250/50 (Item #06-93-2912) is a non-medicated, dry powder inhaler (DPI) simulator designed for use in clinical education and respiratory training. Closely modeled after combination inhalers like Advair Diskus®, this training tool allows students and healthcare providers to safely practice patient instruction and proper inhalation technique—without exposure to active pharmaceutical ingredients.
Clearly labeled For Training Purposes Only, Not for Human or Animal Use, this simulated inhaler is ideal for:
-
Demonstrating correct DPI technique for combination corticosteroid/bronchodilator therapy
-
Teaching breath-activated delivery and timing
-
Practicing patient counseling for asthma and COPD maintenance medications
-
Enhancing skills in respiratory therapy, nursing, and pharmacy education
Used widely in long-term respiratory disease management, the Demo Dose Fluticasone/Salmeterol Disk Inhaler 250/50 provides a realistic and safe platform for clinical simulation, skills labs, and patient education in inhaled combination therapy.
CONTENTS OF TOTES ARE FOR INSTRUCTIONAL USE ONLY. NOT FOR HUMAN OR ANIMAL USE.
---
| Part Number | 06-93-2912 |
|---|---|
| Country of Origin | CN |
| UNSPSC Category | 42301504 |
| Harmonization Code | 9023.00.00.00 |
| Mfg# | Diskus w/zip-lock pouch,label,print box |
| Warranty | No Warranty |